- If a piece of dry silk be briskly rubbed against a warm plate of
polished flint glass, it will be found to have acquired the property of
adhering to it, which it will retain for some seconds; if at the time this
adhesive power exists, the silk and glass be separated from each other,
they will both be found to have gained the property of attracting very
light substances, such as the ashes of paper or fragments of gold leaf;
and the long filaments of the silk, if there be any, will be seen to repel
each other.
- These bodies are said to be electrically excited, and the
phenomena are called electrical phenomena. The peculiar
circumstances under which they occur, are best observed by the use of an
instrument called the electrical machine; it consists of a cylinder of
glass1 supported upon glass pillars, and which
can be made to revolve, so as to press against a cushion of silk rubbed
over with a little amalgam of zinc and mercury; and of two cylinders of
metal, one in contact with the cushion, and the other opposite to the
glass cylinder, both supported upon glass.
- If two gilt pith balls, suspended upon strings of silk covered with
tinsel, be hung upon a wire, placed in contact with either of the metallic
cylinders, and the machine be put in action, the balls will repel each
other; but if one ball be attached to a wire, connected with one metallic
cylinder, and the other ball be attached to a wire connected with the
other, the two balls, when the machine is put into action, will attract
each other; and {92} at the moment that they come in contact, sparks of
light will be perceived, if the experiment be made under favourable
circumstances.
As the two balls, when in contact with the same cylinder, may be
considered as receiving the same impulse of impression, they are said to
be similarly electrified; but when in contact with different
cylinders, they are said to be differently electrified; and
electrified bodies that repel each other, are considered as in the same
electrical states; those that attract each other as in different
electrical states.
- There are probably no two bodies differing in nature, which are not
capable of exhibiting electrical phenomena, either by contact, pressure,
or friction; but the first substances in which the property was observed,
were vitreous and resinous bodies; and hence the different
states were called states of resinous and vitreous
electricity; and resinous bodies bear the same relation to flint glass, as
silk. The terms, negative and positive electricity, have
been likewise adopted, on the idea, that the phenomena depend upon a
peculiar subtile fluid, which becomes in excess in the vitreous, and
deficient in the resinous bodies; and which is conceived by its motion and
transfer, to produce the electrical phenomena.
- Flint glass and silk, silk and sulphur, sulphur and metals, resin and
metals, all by friction or contact, become strongly electrical, and of
course attractive, and communicate their attractive powers to small masses
of matter brought in contact with them; a pith ball, or a slip of gold
leaf that has been touched by flint glass, excited by silk, will be
repelled by a ball or slip that has been touched by silk excited by
sulphur, or by a ball or slip that has been touched by sulphur excited by
{93} metals, so that the attractive and repellent states depend entirely
upon the actions of the two substances, and not upon any power peculiar to
and inherent in each.
- It is upon this circumstance, that the electrometer, which
might be called the differential one, is framed; it consists of two
gold leaves attached to a metallic plate, and included in a hollow
cylinder of glass,2 fixed upon another
metallic plate, which is connected with two pieces of tin foil, pasted
upon the glass opposite to the leaves. When any electrified body is made
to touch the upper plate, the gold leaves diverge; if their divergence is
increased by the approac hof flint glass excited by silk, they are said to
have the same state as the glass, the vitreous or the positive; if their
divergence is diminished, they are said to be in the opposite state, or to
possess the resinous or negative electricity.
- When luminous phenomena are connected with electrical excitation, the
different states may be known by presenting a metallic point to the
excited body; if rays of light issue from the point to the body, it is
said to be negatively electrified: but if the point appears simply
luminous, without sending off any rays, the electricity is said to be
positive.
- For measuring small degrees of electricity of bodies, as compared with
those of others of the same kind, the electrical balance of Coulomb
is applied; it consists of a gilt pith ball, placed upon a metallic rod,
on the opposite extremity of which is a thin leaf of metal; the rod is
suspended horizontally, by a fine metallic wire, which passes into a glass
tube, to the top of which it is attached; the glass tube is inserted into
a cylinder of glass, which contains a copper ball, connected with a small
bar of metal, which is carried through an aperture {94} in the glass
cylinder, into the atmosphere; a very small force only is required to
twist the wire, and when the two balls are brought in contact, and the bar
touched by the electrified body, they gain the same kind of electricity
body, they gain the same kind of electricity, and repel each other; and
the degree of their repulsion may be measured by a scale of degrees, made
on the circumstance of the cylinder.3
- Bodies receive the electrical influence in different manners. If a rod
of glass be brought in contact with any excited electrical body, it will
receive the electrical influence in the part where it touched the body,
and will be electrical, to a little distance, round the point of contact;
but its remote parts will not be affected. A rod of metal, on the
contrary, suspended on a rod of glass, and brought in contact with an
electrical surface, instantly becomes electrical throughout. The glass, in
common philosophical language, is said to be a non-conductor of
electricity, or an insulating substance; the metal a
conductor. Some bodies are affected to a much greater extent than
glass, but not nearly so much as metals; such are animal and vegetable
substances, water, and fluids containing water; they are said to be
imperfect conductors. According to the statements of Mr. Cavendish,
iron conducts 400 million of times better than water, sea water 100 times
better than distilled water, and water saturated with salt, 720 times
better. The mineral acids are the best fluid conducting substances known,
and after them, saline solutions, the powers of which appear to be nearly
in proportion to the quantities of salts they contain. Charcoal and
metals, and the greater number of inflammable metallic compounds are
conductors. Alcohol and ether, are very imperfect conductors; and sulphur,
oils, resinous substances, {95} metallic oxides and compounds of chlorine,
nonconductors.
- There is a stone found in many parts of the world, called tourmaline,
which is sometimes crystallized as a nine-sided pyramid; when this
substance is gently heated, it becomes electrical, and one extremity, that
terminated by the six-sided pyramid, s positive, the other is negative;
to a certain extent, its electricities are exalted by increasing the
temperature; when it begins to cool, it is still found electrical; but the
electricities are changed, the pyramid, before positive, is now negative,
and vice versa. When the stone is of considerable size, flashes of light
may be seen along its surface.
There are other gems and cystallized substances, which possess a property
similar to that of the tourmaline. The luminous appearance of some
diamonds, when heated, probably depends upon their electrical excitation.
The substance called the Boracite, which is a cube, having its edges and
angles defective, becomes electrical by heat, and in one variety presents
no less than eight sides, in different states, four positive, four
negative; and the opposite poles are in the direction of the axes of the
crystal.
- It would appear, that in all cases of electrical action, the two
electrical states are always coincident, either in different parts of the
same body, or in two bodies; and that they are always equal, and capable
of neutralizing each other. If a connection be made by a wire, between
the positive and negative conductors of the electrical machine, during the
time of its action, all electrical effects cease; and to produce a
succession of effects, both conductors must be brought near bodies {96}
connected with the ground, which gain the opposite state, in consequence
of what may be called induction, and which will be explained in the
next paragraph.
- When a non-conductor, or imperfect conductor, provided it be a thin
plate of matter, placed upon a conductor, is brought in contact with an
excited electrical body, the surface, opposite to that in contact, gains
the opposite electricity from that of the excited body; and if the plate
be removed from the conductor and the source of electricity, it is found
to possess two surfaces in opposite states. If a conductor be brought into
the neighbourhood of an excited body, the air, which is a non-conductor,
being between them, that extremity of the conductor, which is opposite to
the excited body, gains the opposite electricity, and the other extremity,
if opposite to a body connected with the ground, gains the same
electricity, and the middle point is not electrical at all. This is easily
proved, by examining the electricity of three sets of gilt pith balls
raised on wires on the different parts of the conductor, which is thus
effected by induced electricity.
If, instead of air, a plate of mica or glass be between the two
conductors, the same phenomena will occur; so that it would appear that
the conductor merely gains two opposite electricities, or polar
electricities, of the same kind as those of the non-conductor. The
phenomena of sparks, of discharges, and of accumulated electricity, depend
upon this law. In the case of the common electrical spark, a stratum of
air is charged in the same manner as a glass bottle, partially coated with
tin-foil, is charged in the Leyden experiment;4 when the hand is held near the positive
conductor of an electrical machine, the person standing on the ground,
{97} the hand is rendered negative, and the states become exalted, till
the polarities, as they may be called, are annihilated through the air,
producing a spark, a snap, and a distinct sensation. If a number of small
pith balls, placed upon a surface of metal, are caused to approach an
electrified body, they are brought into the opposite state by induction,
and are attracted towards the body; but when they come in contact with it,
this state is destroyed, they gain the same state, and are repelled; and
if they are properly placed, their alternate attractions and repulsions
may be produced, as long as the machine is in action.
- If a number of cylinders of metal, insulated on glass, be
placed in a line with each other, but not in contact, and the last be
connected with the ground;5 when a powerfully
electrified conductor of a machine is brought opposite to the first, they
will all become electrical, and every insulated cylinder will present two
poles; the negative pole of one being opposite to the positive pole of
the other; and if a spark is produced by means of the last, sparks occur
throughout the whole arrangement. In like manner a series of Leyden jars
may be made to charge each other, the outer surface of the first rendering
negative the inner surface of the second, and so on; and by connecting the
surfaces that have the same kind of electricity , in the first place, and
then connecting two opposite surfaces in the series, a powerful
explosion6 may be produced.
- When a point connected with the ground is brought near an electrified
substance, it rapidly gains the opposite state, and an immediate discharge
takes place, which continues till the equilibrium is restored. Large
surfaces are electrified by induction much more {98} slowly than small
ones, and are capable of accumulating much more electricity, which renders
the discharge from them much more violent. Indeed, the electrical powers
seem entirely to belong to the surfaces of bodies, and not to be connected
with the quantity of solid matter they contain.
- It is in consequence of the principle of induction, that the
condensing electrometer is so much more sensible than the common
electrometer; this instrument consists of two plates of polished metal,7 the surfaces of which are parallel, one
connected with the plate of the electrometer, the other moveable, in
connexion with the ground, and the plates are very near each other. When
the body supposed to be electrical, is made to touch the top of the
electrometer, and is afterwards removed, in separating the plates, the
effect will be perceived.
- The difference in what are called the conducting powers of bodies,
seems to depend entirely upon the different manner in which they receive
the electrical polarities, or in which their parts become capable of
communicating attractive or repellent powers, to other matter.
Non-conductors appear to receive polarities, only with great difficulty,
but retain them for a long while, and present probably a number of
different alternations of poles, within a small space, and cannot be
affected to any great distance. Imperfect conductors receive polarity
with more facility, but present fewer alternations, and preserve their
electricities for a shorter time. Perfect conductors are easily affected
throughout, but present at most only two poles, and the powers rapidly
destroy each other. The difficulty with which non-conductors receive
polarity, is shewn in the phenomena {99} of charging thick and thin coated
plates of glass and mica. The thin plates are capable of being charged
much more highly than the thick ones, and the accumulation on the
opposite surfaces is much greater.
Rarefied air, or gaseous matter, is much more susceptible of receiving
polarities, than dense air or gaseous matter; and hence, the electrical
spark will pass much further through rarefied air or light gases, than
through dense air or heavy gases; it passes much further, likewise in
gases, than in non-conducting fluids.
- If a non-conducting surface, coated with two conducting surfaces, and
charged so as to give a spark of an inch in length, through air, be
connected by both its conducting surfaces, with a similar apparatus not
charged; then both systems may be discharged together; but the spark they
will give, will be only half as long as the single one would have given,
if discharged alone. The quantity of the electricity in this case,
is conceived not to be altered, but its intensity is said to be
only half as great when it is discharged from a double surface; and these
expressions of intensity and quantity, though it is not easy to attach any
very definite ideas to them, are nevertheless useful, in giving more
facility to the arrangement of some important electrical phenomena.
- When very small conducting surfaces are used for conveying very large
quantities of electricity, they become ignited; and of the different
conductors that have been compared, charcoal is most easily heated by
electrical discharges,8 next iron, platina,
gold, then {100} copper, and lastly zinc. The phenomena of electrical
ignition, whether taking place in gaseous, fluid, or solid bodies, always
seem to be the result of a violent exertion of the electrical attractive
and repellent powers, which may be connected with motions of the particles
of the substances affected. That no subtile fluid, such as the matter of
heat has been imagined to be, can be discharged from these substances, in
consequence of the effect of the electricity, seems probable, from the
circumstance, that a wire of platina may be preserved in a state of
intense ignition in vacuo, by means of the Voltaic apparatus, (an
instrument which will be immediately described,) for an unlimited time;
and such a wire cannot be supposed to contain an inexhaustible quantity of
subtile matter.
- Certain changes in the forms of substances, are always connected with
electrical effects. Thus when vapour is formed or condensed, the bodies in
contact with the vapour, become electrical. If, for instance, a plate of
metal, strongly heated, be placed upon an electrometer, and a drop of
water be poured upon the plate, at the moment the water rises in vapour,
the gold leaves of the electrometer diverge with negative electricity.
Sulphur, when melted, becomes strongly electrical during the time of
congelation; and the case seems to be analogous, with respect to
non-conducting substances in general, when they change their forms.
As electricity appears to result from the general powers or agencies of
matter, it is obvious, that it must be continually exhibited in nature,
and that a number of important phenomena must depend upon its operation.
When aqueous vapour is condensed, the clouds formed are usually more or
less electrical; and the earth below them being brought into an opposite
state, {101} by induction, a discharge takes place when the clouds
approach within a certain distance, constituting lightning; and the
undulation of the air, produced by the discharge, is the cause of
thunder, which is more or less intense, and of longer or shorter duration,
according to the quantity of air acted upon, and the distance of the
place, where the report is heard from the point of the discharge. It may
not be uninteresting to give a further illustration of this idea:
electrical effects take place in no sensible time; it has been found, that
a discharge through a circuit of four miles, is instantaneous; but sound
moves at the rate of about twelve miles in a minute. Now, supposing the
lightning to pass through a space of some miles, the explosion will be
first heard from the point of the air agitated, nearest to the spectator;
it will gradually come from the more distant parts of the course of the
electricity, and last of all, will be heard from the remote extremity; and
the different degrees of the agitation of the air, and likewise the
difference of the distance, will account for the different intensities of
the sound, and its apparent reverberations and changes.
- In a violent thunder storm, when the sound instantly succeeds the
flash, the persons who witness the circumstance, are in some danger: when
the interval is a quarter of a minute, they are secure. In a thunder
storm, the lowest ground is the safest place, and a horizontal posture,
the least dangerous; the neighbourhood of trees, or buildings, should be
avoided, particularly of trees, the living juices of which are calculated
to conduct the electricity, and make part of a circuit. In a house, the
celld ars are the safest places, and in a room the person should stand as
far as possible from the fire. The means adopted by Franklin have however,
to a {102} great extent, averted the destructive effects of atmospheric
electricity; and by pointed conductors, the thunder cloud is disarmed of
its terrors, and the lightning slowly discharged in harmless
coruscations.
If a school-boy's kite be mounted high in the atmosphere, by means of a
string, containing filaments of metal, fastened to a conductor, fixed on a
glass rod; the conductor usually gives signs of electricity, which will be
greatest, when clouds are floating in the atmosphere; and it was by means
of a simple apparatus of this kind, that the American Philosopher effected
his grand discovery of the identity of electricity and lightning.
The water-sprout is probably the result of the operation of a weakly
electrical cloud, at an inconsiderable elevation above the sea, brought
into an opposite state: and the attraction of the lower part of the cloud,
for the surface of the water, may be the immediate cause of this
extraordinary phenomenon.
The coruscations of the Aurora Borealis, and Australis, precisely resemble
strong artificial electricity, discharged through rare air; and as the
poles are non-conductors, being coated with ice or snow, and as vapour
must be constantly formed in the atmosphere above them; the idea of
Franklin is not improbable, that the Auroras may arise from a discharge of
electricity, accumulated in the atmosphere near the poles, into its rarer
parts; through other solutions of the phenomena may be given on the idea,
that the earth itself is endowed with electrical polarity; or that the
motions of the atmosphere produce the effect: but all views on this
subject must be hypothetical, and the light may result from other causes
than electrical action.
- The common exhibition of electrical effects, is in {103} attractions
and repulsions, in which masses of matter are concerned; but there are
other effects, in which the changes that take place, operate in a manner,
in small spaces of time imperceptible, and in which the effects are
produced upon the chemical arrangements of bodies.
If a piece of zinc and a piece of copper be brought in contact with each
other, they will form a weak electrical combination, of which the zinc
will be positive, the copper negative; this may be learnt by the use of a
delicate condensing electrometer; or by pouring zinc filings through
holes, in a plate of copper, upon a common electrometer; but the power of
the combination may be most distinctly exhibited in the experiments,
called Galvanic experiments, by connecting the two metals, which
must be in contact with each other, with a nerve and muscle in the limb of
an animal recently deprived of life, a frog for instance; at the moment
the contact is completed, or the circuit made, one metal touching the
muscle, the other the nerve, violent contractions of the limb will be
occasioned. If a piece of zinc and copper, in contact with each other in
one point, be placed in contact in other points with the same portion of
water; the zinc will corrode and attract oxygen from the water, much more
rapidly than if it had not been in contact with the copper; and if a
small quantity of sulphuric acid be added to the water, it will be seen
that globules of inflammable air are given off from the copper, though it
is not dissolved nor acted upon.
- The connection of chemical effects, with the exhibition of electrical
powers is, however, best witnessed in combinations, in which these powers
are accumulated by alternations of different metals and {104} fluids. If
plates of copper and zinc two or three inches square, and pieces of cloth
of the same size soaked in a solution of salt, or sal ammoniac, or nitre,
be arranged in the order of copper, zinc, moistened cloth, and so on, and
made into an insulated pile, of which the series are 200;9 several remarkable phenomena will occur.
When one hand is applied to the bottom of the pile, and the other to the
top, both hands being moistened, a shock will be perceived.
When a metallic wire, having a bit of well burned charcoal at its
extremity, is made to connect the two extremities of the pile, a spark
will be perceived, or the point of the charcoal will become ignited.
A wire, connected with the top of the pile, brought in contact with a
sensible electrometer, will cause the leaves to diverge; and by removing
the wire and applying excited glass to the electrometer, it will be found
that the electricity is positive; a wire connected with the bottom of the
pile will affect it with negative electricity; a wire from the middle of
the pile will have no influence on the instrument.
If wires of platina from the extremities of the pile be introduced
into water, or into two portions of water connected by moist substances,
oxygen gas will separate at the wire exhibiting the positive electricity,
and hydrogen gas at the wire exhibiting the negative electricity; and the
proportions are such, when the proper circumstances exist, that they will
produce water when exploded by the electrical spark, that is, the volume
of hydrogen will be to that of oxygen as two to one.
If the same wires be introduced into a strong solution of sulphuric or
phosphoric acid, or into metallic solutions, oxygen will separate at the
positive surface, the {105} inflammable or metallic matter contained in
the solution at the negative surface.
When any substance rendered fluid by heat, consisting of water, oxygen and
inflammable or metallic matter, is exposed to those wires, similar
phenomena occur.
When any solution of a neutral salt containing acid, united to alkaline,
earthy, or common metallic matter, is used; besides the other phenomena
that take place, acid matter collects round the positively electrified
surface; alkali, earth, or oxide, round the negative surface; and if two
separate vessels are employed to contain the solution, connected by moist
asbestos, it is found that the acid collected in the vessel containing the
wire, positively electrified, will be in definite proportion to the matter
collected in the other cup; that is, it will form with it a neutrosaline
compound.
If a solution of muriatic acid in water be acted on by the wires, hydrogen
will separate at the negative surface, and chlorine at the positive
surface.
- This apparatus, which exhibits in so distinct a manner the relations
of electrical polarities to chemical attractions, is the grand invention
of Volta, made known in the first year of this century; its electrical
effects have been long known, but the phenomena of its operation in
decomposing bodies, are of more recent discovery.
Several modes of constructing it have been adopted, some of which are much
superior in point of convenience, to that which has been just
described.
One mode is by soldering the plates of zinc and copper together, and by
cementing them into troughs of baked wood, covered with cement, in the
regular order, so as to form cells to be filled with the fluid menstruum;
{106} each surface of zinc being opposite to a surface of copper; and
this combination is very simple and easy of application.
Another form is that of introducing plates of copper and of zinc, fastened
together by a slip of copper, into a trough of porcelain containing a
number of cells corresponding to the number of the series. The different
series may be introduced separately into the troughs, and taken out
without the necessity of changing the fluid, or they may be attached to a
piece of baked wood and (when the number is not very large) introduced
into the cells, or taken out together.10
- Similar polar electrical arrangements to those formed by zinc and
copper, may be made by various alternations of conducting and imperfect
conducting substances; but for the accumulation of the power, the series
must consist of three substances or more, and one at least must be a
conductor. Silver or copper, when brought in contact with a solution of a
compound of sulphur and potassa, at one extremity, and in contact with
water or a solution of nitric acid, at the other extremity, some saline
solution being between the sulphuretted and the acid solutions, forms an
element of a powerful combination, which will give shocks when fifty are
put together; the order is copper, cloth of the same size moistened with
solution of nitric acid, cloth moistened in solution of common salt, cloth
moistened in solution of the compound of sulphur, copper, and so on; the
specific gravities of the solutions should be in the order in which they
are arranged, to prevent the mixture of the acid and sulphuretted
solution; that is, the heaviest solution should be placed lowest.
The tables annexed contain some series, which form {107} Voltaic
electrical combinations, arranged in the order of their powers; the
substance most active being named first in each column.
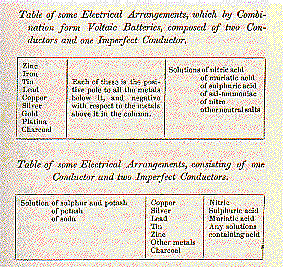 The metals having the strongest attraction for oxygen, are the metals
which form the positive pole, in all cases in which the fluid menstrua act
chemically by affording oxygen; but when the fluid menstrua afford sulphur
to the metals, the metal having the strongest attraction for sulphur
under the existing circumstances, determines the positive pole; thus in a
series of copper and iron, introduced into a porcelain trough, the cells
of which are filled with water or with {108} acid solutions, the iron is
positive, and the copper negative; but when the cells are filled with
solutions of sulphur and potash, the copper is positive and the iron
negative.
The metals having the strongest attraction for oxygen, are the metals
which form the positive pole, in all cases in which the fluid menstrua act
chemically by affording oxygen; but when the fluid menstrua afford sulphur
to the metals, the metal having the strongest attraction for sulphur
under the existing circumstances, determines the positive pole; thus in a
series of copper and iron, introduced into a porcelain trough, the cells
of which are filled with water or with {108} acid solutions, the iron is
positive, and the copper negative; but when the cells are filled with
solutions of sulphur and potash, the copper is positive and the iron
negative.
In all combinations in which one metal is concerned, the surface opposite
the acid is negative, that in contact with solution of alkali and sulphur,
or of alkali, is positive.
- The energy of a combination to give repulsive or attractive powers to
masses of matter or to affect the electrometer, seems to increase with the
number of the series, as does the power to give shocks, and to decompose
bodies; but as long as the surface of the gold leaves in the electrometer,
or of the human body, or of the water acted upon, is the same, and less
than that of the acting plates, increase of surface of the plates is
connected with no increase of power. In the operation upon metallic
substances or charcoal, or upon good imperfect conductors, the case,
however, is different. Thus, though a battery composed of plates of copper
and zinc a foot square, will not affect the condensing electrometer more,
nor decompose more water, nor give greater shocks to the fingers, than a
battery containing plates of an inch square, yet it will ignite more than
100 times as much fine platina wire, and decompose sulphuric acid, and the
water in strong saline solutions with infinitely more rapidity. This has
been expressed by Mr. Cavendish in the statement, that the intensity is
the same in both cases; but that the quantity is in some ratio as the
surface. The quantity in the small plates is as much or more than such
imperfect conductors as water and the human body can carry off by a small
surface: whilst better conductors can transmit the whole quantity afforded
by the large plates, even when used {109} in the very thin laminae or
wires. The correctness of this view may be shewn by a very simple
experiment. Let two platina wires, from the extremities of a battery
composed of plates of a foot square, be plunged into water, the quantity
of gas disengaged from the wires will be nearly the same as from an equal
number of plates of an inch square; let the fingers of each hand,
moistened with water, be applied to the two extremities of the battery, a
shock will be perceived nearly the same as if there had been no connection
between the wires and the water. Whilst the circuit exists through the
human body and through water, let a wire attached to a thin slip of
charcoal be made to connect the two poles of the battery, the charcoal
will become vividly ignited. The water and the animal substance discharge
the electricity of a surface, probably not superior to their own surface
of contact with the metals; the wires discharge all the residual
electricity of the plates; and if a similar experiment be made on plates
of an inch square, there will scarcely be any sensation, when the hands
are made to connect the ends of the battery, a circuit being previously
made through water; and no spark when charcoal is made the medium of
connection, imperfect conductors having been previously applied.
The first distinct experiment upon the igniting powers of large plates was
performed by MM. Fourcroy, Vauquelin, and Thenard. But the grandest
combination over constructed for exhibiting the effects of extensive
surface, was made by Mr. Children: it consists of twenty double plates
four feet by two; of which the whole surfaces are exposed, in a wooden
trough, in cells covered with cement, to the action of diluted acids. This
battery, when in full action, had no more {110} effect on water or on the
human body than one containing an equal number of small plates; but when
the circuit was made through metallic wires, the phenomena were of the
most brilliant kind. A platina wire of one-thirtieth of an inch in
thickness, and eighteen inches long, placed in the circuit between bars of
copper, instantly became red hot, then white hot, the brilliancy of the
light was soon insupportable to the eye, and in a few seconds the metal
fell fused into globules. The other metals were easily fused or dissipated
in vapour by this power. Points of charcoal ignited by it produced a
light so vivid, that even the sunshine compared with it appeared
feeble.
Mr. Children has another battery in construction, the plates of which are
double the size of that just described, and which are to be arranged in
pairs in single troughs, and connected by means of plates of lead in
regular order.11
- The most powerful combination that exists in which number of
alternations is combined with extent of surface, is that constructed by
the subscriptions of a few zealous cultivators and patrons of science, in
the laboratory of the Royal Institution. It consists of two hundred
instruments, connected together in regular order, each composed of ten
double plates arranged in cells of porcelain, and containing in each plate
thirty-two square inches; so that the whole number of double plates is
2000, and the whole surface 128,000 square inches. This battery, when the
cells were filled with 60 parts of water mixed with one part of nitric
acid, and one part of sulphuric acid, afforded a series of brilliant and
impressive effects. When pieces of charcoal about {111} an inch long and
one-sixth of an inch in diameter, were brought near each other (within the
thirtieth or fortieth part of an inch,) a bright spark was produced, and
more than half the volume of the charcoal became ignited to whiteness, and
by withdrawing the points from each other a constant discharge took place
through the heated air, in a space equal at least to four inches,
producing a most brilliant ascending arch of light, broad, and conical in
form in the middle.12 When any substance was
introduced into this arch, it instantly became ignited; platina melted as
readily in it as wax in the flame of a common candle; quartz, the
sapphire, magnesia, lime, all entered into fusion; fragments of diamond,
and points of charcoal and plumbago, rapidly disappeared, and seemed to
evaporate in it, even when the connection was made in a receiver exhausted
by the air pump; but there was no evidence of their having previously
undergone fusion.
When the communication between the points positively and negatively
electrified was made in air, rarefied in the receiver of the air pump, the
distance at which the discharge took place increased as the exhaustion was
made, and when the atmosphere in the vessel supported only one-fourth of
an inch of mercury in the barometrical gage, the sparks passed through a
space of nearly half an inch; and by withdrawing the points from each
other, the discharge was made through six or seven inches, producing a
most beautiful coruscation of purple light, the charcoal became intensely
ignited, and some platina wire attached to it, fused with brilliant
scintillations, and fell in large globules upon the plate of the pump. All
the phenomena of chemical decomposition were produced with intense
rapidity by this {112} combination. When the points of charcoal were
brought near each other in non-conducting fluids, such as oils, ether, and
oxymuriatic compounds, brilliant sparks occurred, and elastic matter was
rapidly generated; and such was the intensity of the electricity, that
sparks were produced, even in good imperfect conductors, such as the
nitric and sulphuric acids.
When the two conductors from the ends of the combination were connected
with a Leyden battery, one with the internal, the other with the external
coating, the battery instantly became charged, and on removing the wires,
and making the proper connections, either a shock or a spark could be
perceived; and the least possible time of contact was sufficient to renew
the charge to its full intensity.
- The general facts of the connection of the increase of the different
powers of the battery with the increase of the number and surface of the
series, are very distinct; but to determine the exact ratio of the
connection is a problem not easy of solution.
MM. Gay Lussac and Thenard have announced, that the power of chemical
decomposition increases only as the cube root of the number of plates; but
their experiments were made with parts of piles of a construction very
unfavourable for gaining accurate results; and in various trials made with
great care in the laboratory of the Royal Institution, the results were
altogether different. The batteries employed were parts of the great
combination, carefully insulated, and similarly charged; arcs of zinc and
silver presenting equal surfaces, and arranged in equal glasses filled
with the same kind of fluid, were likewise used; and the tubes for
collecting the gases were precisely similar, and filled with the same
solution of potassa.13 In these experiments
ten {113} pairs of plates produced fifteen measures of gas; twenty pairs
in the same time produced forty-nine: again, ten pairs produced five
measures; forty pairs in the same time produced seventy-eight measures. In
experiments made with arcs, and which appeared unexceptionable, four pairs
produced one measure of gas; twelve pairs in the same time produced nine
and 7/10 of gas; six pairs produced one measure of gas; thirty pairs,
under like circumstances, produced 24.5 measures; and these quantities are
nearly as the squares of the numbers.
It would appear from the experiments of Vanmarum and Pfaff, confirmed by
those of Messrs. Wilkinson, Cuthbertson, and Singer, that the increase of
power of batteries, the plates of which have equal surfaces, is as the
number. I found that ten double plates, each having a surface of a
hundred square inches, ignited two inches of platina in wire of
one-eightieth of an inch; twenty plates, five inches; forty plates, eleven
inches: but the results of experiments on higher numbers were not
satisfactory; for one hundred double plates of thirty-two square inches
each, ignited three inches of platina wire of one-seventieth, and one
thousand ignited only thirteen inches, and the charges of diluted acid
were similar in both cases.
The power of ignition for equal numbers of plates, seems to increase in a
very high ratio with the increase of surface, probably higher than even
the square; for twenty double plates, containing each two square feet, did
not ignite one-sixteenth as much wire as twenty, containing each eight
square feet, the acid employed being of the same strength in both
cases.
Numerous circumstances are opposed to the accuracy of experiments made
with high numbers, or very large surfaces; the activity of combinations
rapidly {114} diminishes in consequence of the decomposition of the
menstruum used; and this decomposition is much more violent, the greater
the number and surface of the alternations; the vapour rising likewise,
when the action is intense, interferes by its conducting powers, and the
gas by its want of conducting power; and when series containing above five
hundred double plates are used, unless the insulation is very perfect,
there is a considerable loss of electricity; thus the great battery of two
thousand double plates belonging to the Royal Institution, will scarcely
act by its true poles, when arranged on a floor of stone, and requires not
merely the insulation of porcelain, but likewise of dry wood; and when
arranged on a stone floor, it is hardly possible to walk near any of the
approaching series without receiving shocks. In cases of the ignition of
wire, the cooling influence of the substances in contact, and of that part
of the chain not ignited, interferes most, when small quantities of wire
are employed, and with feeble powers; and hence the effect is at first in
a lower and then in a higher ratio than the number, when the whole range
is small, as in the experiments above stated. If there is an imperfect
connection in any of the series, a great diminution of power is the
consequence. If one plate is corroded, or covered with more oxide than the
rest, there is a general loss of effect. If copper is substituted for
zinc, or zinc for copper, in a single series, the result is similar; and I
find that a platina wire, introduced in the place of an arc of silver and
zinc, in a series of thirty, diminished its power of producing gas so
much, that it was equal only to that of four.
- The circumstance most important in electricity, perhaps, is its
connection with the chemical powers of matter, and the manner in which it
modifies, exalts, or {115} destroys these powers. Most of the substances
that act distinctly upon each other electrically, are likewise such as
act chemically, when their particles have freedom of motion: this is the
case with the different metals, with sulphur and the metals, with acid and
alkaline substances; and the relations of bodies are uniform; those that
have the highest attracting powers being in the relation of positive, in
arrangements in which chemical changes can go on. Thus, as is shown in the
tables, page 107, zinc is positive with respect to iron, iron with respect
to copper, copper with respect to silver, and so on in all combinations in
which oxygen is capable of being combined with the metal; but copper is
positive with respect to iron in compound menstrua containing sulphur; the
electrical power being in all cases apparently connected with the power
of chemical combination.
Crystals of oxalic acid touched by dry quick-lime exhibit electrical
powers; and the acid is negative, the lime positive.
All the acid crystals, upon which I have experimented when touched by a
plate of metal, render it positive. And in Voltaic combinations with
single plates or arcs of metal, as is stated in page 108, the metal is
negative on the side opposed to the acid, and positive on the side or pole
opposed to the alkali.
Bodies that exhibit electrical effects previous to their chemical action
on each other, lose this power during combination. Thus, if a polished
plate of zinc is made to touch a surface of dry mercury, and quickly
separated, it is found positively electrical, and the effect is increased
by heat; but if it be so heated as to amalgamate with the surface of the
mercury, it no longer exhibits any marks of electricity. The case is
analogous {116} with copper and sulphur; and iron acts more powerfully
than zinc with quicksilver in a permanent electrical combination, as in
the experiments of Colonel Haldane; apparently, because under common
circumstances it is incapable of amalgamating with that metal. When any
conducting substance, capable of combining with oxygen, has its positive
electricity increased, it will attract oxygen with more energy from any
imperfect conducting medium; and metallic bodies which in their common
state have no action upon water, such as silver, attract oxygen from it
easily, when connected with the positive pole in the Voltaic circuit; and
bodies that act upon water, such as zinc and iron, so as to decompose it
slowly, refuse to attract oxygen from it when they are negatively
electrified in the Voltaic circuit.
Acids, which are negative with respect to alkalies, metals, and earths,
are separated from these bodies in the Voltaic circuit at the positive
surface; and alkalies, metals, and earths, are separated from acids at the
negative surface: and such are the attracting powers of these surfaces,
that acids are transferred through alkaline solutions, and alkalies
through acid solutions, to the surfaces where they have their points of
rest. It is easy to shew this by making a combination of three agate
cups,14 one containing sulphate of potassa,
one weak nitric acid, and the third distilled water, and connecting them
by asbestus moistened in pure water, in such a manner, that the surface of
the acid is lower than the surface of the fluid in the other two cups.
When two wires of platina from a powerful Voltaic apparatus are introduced
into the two extreme cups, the solution of the salt being positively
electrified, a decomposition will take place, and in a certain time a
{117} portion of potassa will be found dissolved in the cup in contact
with the negative wire, though the fluid in the middle cup will still be
sensibly acid.
- Such are the decomposing powers of electricity, that not even
insoluble compounds are capable of resisting their energy; for even glass,
sulphate of baryta, flour spar, &c. when moistened and placed in contact
with electrified surfaces from the Voltaic apparatus, are slowly acted
upon, and the alkaline, earthy, or acid matter carried to the poles in the
common order. Not even the most solid aggregates, nor the firmest
compounds, are capable of resisting this mode of attack; its operation is
slow, but the results are certain; and sooner or later, by means of it,
bodies are resolved into simpler forms of matter.
- It is in consequence of the phenomena of electrical decomposition, in
which metals, inflammable bodies, alkalies, earths, and oxides, are
determined to the negative surface, and oxygen, chlorine, and acids, to
the positive surface, that for some time it was conceived, that various
substances, might be composed from pure water, by means of electricity,
such as potassa, soda, and muriatic acid. A strict investigation of the
circumstances under which these substances appeared, led me to discover
that they were always furnished from the vessels, or from impurities in
the water, and enabled me to determine the general principles of
electrical decomposition, and to apply this power to the resolution of
some species of matter, of unknown nature, into their elements.
- The connection of electrical phenomena and chemical changes is evident
likewise in the general phenomena of the battery. The most powerful
Voltaic combinations are formed by substances that act chemically {118}
with most energy upon each other; and such substances as undergo no
chemical changes in the combination, exhibit no electrical powers. Thus,
zinc copper, and nitric acid form a powerful battery; whilst silver, gold,
and water, which do not act chemically on each other, in series of the
same number, produce no sensible effect. These circumstances led some
philosophers to suppose, at an early period of the investigation of the
electrical powers of metals, that they were entirely the result of
chemical changes: that as heat was produced by this action, when exerted
under common circumstances, so electricity resulted from it under other
circumstances; and many of the phenomena were comfortable to such an idea,
and some ingenious inquirers adopted it to such an extent, as to suppose
electricity in all cases owing to this cause.
This generalization, whether applied to Voltaic or to common electricity,
seems, however, to be incorrect. Zinc and copper, as has been stated,
different metals and oxalic acid, different metals and sulphur, or
charcoal, exhibit electrical effects after mere contact, and that in cases
when not the slightest chemical change can be observed; and if in these
experiments chemical phenomena are produced by the action of menstrua, all
electrical effect immediately cease: and it is not philosophical to assume
a cause to account for an effect, when no such case can be perceived.
It has been supposed that the action of the common electrical machine
depends upon the oxidation of the amalgam; but I found by mounting a small
machine in a glass vessel, in such a manner that it could be made to
revolve in any species of gas, that it was active in hydrogen gas, and
more active in carbonic acid gas than in the atmosphere (probably owing to
its greater {119} density). The experiment has been several times repeated
under different circumstances, and uniformly with the same results; and
may be regarded as decisive in this important question.
- Electrical effects are exhibited by the same bodies, when acting as
masses, which produce chemical phenomena when acting by their particles;
it is not therefore improbable, that the primary cause of both may be the
same, and that the same arrangements of matter, or the same attractive
powers, which place bodies in the relations of positive and negative, i.e.
which render them attractive of each other electrically, and capable of
communicating attractive powers to other matter, may likewise render their
particles attractive, and enable them to combine, when they have full
freedom of motion.
It is not a little in favour of this hypothesis, that heat, and sometimes
heat and light, result from the exertion of both electrical and chemical
attractive powers; and that by rendering bodies, which on contact are in
the relation of positive to others, still more highly positive, as has
been stated, page 116, their powers of combination are increased; whereas,
when they are placed in a state corresponding to the negative electrical
state, their powers of union are destroyed. That acids can be detached
from alkalies, oxygen and chlorine from inflammable matter by metallic
substances, or by a fluid menstruum highly positive, is likewise
favourable to the supposition.
- This view of the possibility of the dependence of electrical and
chemical action upon the same cause, has been much misrepresented. It has
been supposed that the idea was entertained, that chemical changes were
occasioned by electrical changes; than which {120} nothing is further from
the hypothesis which I have ventured to advance. They are conceived, on
the contrary, to be distinct phenomena; but produced by the same
power, acting in one case on masses, in the other case on particles.
The hypothesis has been attempted to be controverted by experiments which
are far from satisfactory, and some of which have no connection with it.
It has been said that acids rendered positive by the common machine, will
still combine with alkalies, and that other contradictory results may be
obtained; but a non-conducting acid, though brought in contact with a
positive surface, electrified by the common machine, is not rendered
positive throughout; but gains a polar electricity, which extends only to
a certain depth into the crystals, and the exterior surface, if electrical
at all, is negative: and if a wire, positively electrified by the common
machine, be introduced into an acid solution, this solution, if at all
affected, when made to act upon another solution, will be negative at its
point of action; that is, it will be positive near the wire, but will be
in the opposite state with regard to another surface. And common
electricity is too small in quantity, in its usual form of application, to
influence chemical changes; for it requires a very strong machine acting
upon a very small surface, to produce any sensible polar decompositions of
bodies.
- The power of action of the Voltaic apparatus, seems to depend upon
causes similar to those which produce the accumulation in the Leyden
battery, namely, the property of non-conductors and imperfect conductors
to receive electrical polarities from, and to communicate them to
conductors; but its permanent action is connected with the decomposition
of the chemical menstrua between the plates. Each plate of {121} zinc is
made positive, and each plate of copper negative, by contact; and all the
plates are so arranged with respect to each other as to have their
electricities exalted by induction, so that every single polar
arrangement, heightens the electricity of every other polar arrangement;
and the accumulation of power increases with the number of the series.
When the battery is connected in a circle, the effects are demonstrated by
its constant exhibition of chemical agencies, and the powers exist as long
as there is any menstruum to decompose: but when it is insulated, and the
extreme poles of zinc and copper are unconnected, no effects whatever are
perceived to take place, no chemical changes go on, and it exhibits its
influence only by communicating very weak charges to the electrometer, the
end terminated by zinc communicating a positive charge, that terminated by
copper, a negative charge.
That each plate of the most oxidable metal in the apparatus, is in the
relation of positive, and each plate of the least oxidable, in the
relation of negative, and that every series is possessed of similar and
equal polarity, is shewn by a very simple experiment; forty rods of zinc
of the same size, connected with forty silver wires precisely similar,
were introduced in the regular order into similar glasses filled with a
solution of muriate of ammonia, rendered slightly acid by muriatic acid;
as long as the extreme parts remained unconnected, no gas was disengaged
from the silver, and the zinc was scarcely acted upon; when they were
connected, all the plates of zinc were dissolved much more rapidly, and
hydrogen gas was evolved from every silver wire. And in another
experiment, in which several of these wires at equal distances were
introduced {122} into small glass tubes, it was found that equal
quantities of hydrogen were produced.
- It seems absolutely necessary for the exhibition of the powers of the
Voltaic apparatus, that the fluid between the plates should be susceptible
of chemical change, which appears to be connected with the property of
double polarity, of being rendered positive at one surface, and negative
at the other. There are substances that are imperfect conductors, which
are capable of receiving only one kind of electricity, when made parts of
the Voltaic circuit, and which M. Ehrman, who discovered them, has named
unipolar bodies. Perfectly dry soap, and the flame of phosphorus,
when connected with the two extremities of the Voltaic apparatus, and with
the ground, discharge only the negative electricity. The flames of
alcohol, hydrogen, wax, and oil, discharge under like circumstances only
the positive electricity; but all these bodies when connected with one
pole only of the pile, and with the ground, destroy the divergence of the
leaves of the electrometer connected with that end. It is not difficult to
exhibit these phenomena when the atmosphere is dry, by means of two
hundred pair of plates carefully insulated: an insulated gold leaf
electrometer having a moveable wire attached to it, should be connected
with each end of the pile: when either electrometer is brought in contact
with soap, the soap being connected with the ground, the slight divergence
of the leaves will cease; when the soap is connected with both
electrometers and with the ground, the divergence of the leaves of the
electrometer connected with the end terminated by the zinc, will
continue, the leaves of the other electrometer will collapse. The opposite
effect {123} occurs when the flame of a taper is connected with both
electrometers and with the ground.
The unipolar conductors are incapable of being active in any part of the
pile, and in this respect agree with non-conductors; many of which, it is
probable, if examined in their relations to electricities of low
intensity, would exhibit similar differenc es.
- There are no fluids known, except such as contain water, which are
capable of being made the medium of connection between the metals, or
metal of the Voltaic apparatus; and in cases in which Voltaic batteries
have been said to be constructed by metals and paper, or metals and
starch, or other like substances, the feeble effects produced are merely
owing to the small quantity of water adhering to these substances, which
will not act when carefully dried. The instrument, called by M. de Luc,
the electrical column, formed of zinc, Dutch leaf, and paper, and which
he appears to consider as a different combination from the pile of Volta,
seems to be merely a feeble Voltaic apparatus, in which the quantity of
electricity is not sufficiently great to produce any chemical changes, or
distinct phenomena of ignition; but in which the intensity of the small
quantity existing, when the combination amounts to 400 or 500, is
sufficient to enable it to affect the electrometer, and to act through a
plate of air.
It is very probable that the power of water to receive double polarities,
and to evolve oxygen and hydrogen, is necessary to the constant operation
of the connected apparatus; and that acids, or saline bodies, increase the
action, by affording elements which possess opposite electricities to
each other, when mutually excited; the action of the chemical menstrua
exposes continually {124} new surfaces of metal; and the electrical
equilibrium may be conceived in consequence, to be alternately destroyed
and restored, the changes taking place in imperceptible portions of
time.
The manner in which aqueous fluids receive and communicate electrical
polarity, is shewn by a very simple experiment; let a number of fine
metallic surfaces, or flattened wires (of tin for instance) be made to
swim in a narrow trough containing water; and let two wires from the
extermities of a Voltaic battery of 1000 double plates, be plunged into
the remote ends of the trough, one into one end, the other into the other
end. The metals swimming on the water will immediately acquire electrical
polarity; and the positive and negative poles will be regularly opposed to
each other, the pole of the metal opposite to the wire positively
electrified, will be found to be negative, giving off hydrogen, the other
pole will deposit oxide; the next wire to this will present the alternate
order, which will be preserved in all of them; those most remote from the
right line of the circuit, will be least affected. If the battery be in a
highly active state, the different wires will attract each other by their
opposite poles, and the circle will at length be closed with the
production of brilliant sparks. The phenomena are precisely analogous to
those phenomena in magnetism, presented by a number of flattened
wires of soft iron, made to swim upon water, and rendered magnetic by the
opposite poles of two powerful magnets; each wire has a north pole and a
south pole; and in the alternation, the different poles are attractive of
each other.
- That the decomposition of the chemical agents is connected with the
energies of the pile, is evident from all the experiments that have been
made; as yet no {125} sound objection has been urged against the theory
that the contact of the metals destroys the electrical equilibrium, and
that the chemical changes restore it; and in consequence that the action
exists as long as the decompositions continue; and this conclusion is
confirmed by the late researches made by MM. Gay Lussac and Thenard, on
the great pile constructed by order of the French government. The manner
in which chemical changes tend to restore the electrical equilibrium, is
shewn by a remarkable experiment on the electrization of mercury, which I
have very lately made. A few globules of mercury are placed in a vessel
containing common pump water; or any water that contains a small quantity
of saline impregnation; wires from a battery of 1000 double plates, not
very strongly charged, are introduced into the vessel opposite to each
other, so as to reach the bottom; as soon as the circle is completed, the
mercury will be violently agitated, each globule will become elongated
towards the positive pole, but will retain its circular outline in the
part opposite to the negative pole; oxide will be given off from this
part, which is positive, but no hydrogen from the part which is negative,
and the oxide will pass in a rapid current from the positive towards the
negative pole. As long as no hydrogen is given off, the globule is in
continued agitation, and a stream of oxide flows with great rapidity from
the positive to the negative surfaces; and the negative surfaces of the
mercury approach rapidly towards the positive, which are at rest; if the
conducting power of the water is exalted by the addition of more of the
saline impregnation, or if the charge of the battery be increased,
hydrogen will be given off from the negative poles; and the instant this
happens the globules become {126} stationary; as if the same power which
gave motion to the mercury was neutralized by, or employed in, the
evolution of the hydrogen. There are many other remarkable phenomena
connected with the operation of electricity on mercury, in contact with
water; which may be urged in favour of the idea, that chemical and
electrical attraction depend upon the same cause, and which will possibly
lead to new views respecting the elements of matter; but the consideration
of them properly belongs to a more advanced division of this work.
- The illustrious inventor of the new electrical apparatus, has given it
the name of the electromotive apparatus, and has founded his theory of its
operation upon the Franklinian idea of an electrical fluid, for which
certain bodies have stronger attractions than others: and he conceives,
that in his pile the upper plate of zinc attracts electricity from the
copper, the copper from the water, the water again from the next plate of
zinc, the next plate of zinc from the next plate of copper, and so on.
This hypothesis applies very happily to most of the phenomena of the
action of the insulated pile, and the pile connected by either of its
extremities with the ground; but does not explain with the same facility,
the powers of the apparatus connected in a circle, in which each plate of
zinc must be supposed to have the same quantity of electricity as each
plate of copper; for it can only receive as much as the copper can give,
unless indeed the phenomena of the circular apparatus be considered as
depending upon the constant and rapid circulation of the natural quantity
of electricity, in the different series; which requires the proof of a
constant power in a substance to attract electricity from {127} one body,
at the same time that it is giving it off, to an other.
- Whatever may be the happiest approximation to the
true theory of the Voltaic instrument, it can scarcely be doubted that the
electrical organs of certain animals depend upon similar arrangements of
exciting bodies. The shock of the Gymnotus Electricus, and the
Torpedo, resemble the Voltaic shock: and the power resides in
organs which consist of a number of similar alternations of different
substances. The effects are analogous to those which a Voltaic apparatus
of small surface, consisting of very numerous but not very powerful
series, would produce. It has been conceived that other phenomena of
living action may be connected with the operation of weak electrical
powers; such as secretion; and some ingenious hints on this subject have
been advanced by Dr. Wollaston and Sir Everard Home, and some experiments
relating to the subject instituted by Mr. Brande. Such inquiries are
worthy of further pursuit, as they may tend to elucidate some important
functions of the animal economy; but they must not be confounded with
certain vague speculations, that have been advanced by some authors, on
the general dependence of nervous or sensitive action, and muscular or
irritable action, upon electricity; such speculations are mere
associations of words derived from known phenomena, and applied
illogically to unknown things. The laws of dead and living nature appear
to be perfectly distinct: material powers are made subservient to the
purposes of life, and the elements of matter are newly arranged in living
organs; but they are merely the instruments of a superior principle.
As electrical changes are almost constantly taking {128} place in the
atmosphere, and as the different substances composing the exterior of the
globe, bear different electrical relations to each other, it is very
probable that many of the chemical changes taking place on the surface,
are influenced by the action of weak electrical powers: such as the
decomposition of the surfaces of rocks, the modifications of soils, the
formation of acid, and development of alkaline compounds; and the mutual
agencies of the elements in the earth, the sea, and the atmosphere, may be
assisted or modified by the circumstances of general electrical action.
- With regard to the great speculative questions, whether the electrical
phenomena depend upon one fluid, in excess in the bodies positively
electrified, and in deficiency in the bodies negatively electrified, or
upon two different fluids, capable by their combination of producing heat
and light, or whether they may be particular exertions of the general
attractive powers of matter, it is perhaps impossible to decide in the
present imperfect state of our knowledge. The application of electricity
as an instrument of chemical decomposition, and the study of its effects,
may be carried on independent of any hypothetical ideas concerning the
origin of the phenomena; and these ideas are dangerous only when they are
confounded with facts. Some modern writers have asserted the existence of
an electrical fluid with as much confidence as they would assert the
existence of water, and have even attempted to shew that it is composed of
several other elements; but it is impossible in sound philosophy to adopt
such hasty generalizations; Franklin, Cavendish, Epinus, and Volta, the
illustrious advocates for the idea of a single electrical fluid, have
advanced it only as hypothetical, as accounting in a happy way for most of
the {129} phenomena; and none of the facts that have been brought forward
in favour of the actual existence either of one or two fluids, can be
considered as conclusive.
From a very ingeniously contrived experiment of Mr. Cuthbertson, it
appears that when a stream of electrical sparks is passed through the
flame of a candle between two electrified surfaces, the surface which is
negative is most heated; and it has been argued that a current must pass
from the positive surface to the negative.
But it is more probable, that this phenomenon depends upon the positive
unipolar quality of the flame of wax or tallow referred to above;
for supposing this flame to become positive, which would seem to be the
case, it must be attracted by the negative, and not by the positive
surface; and this view is confirmed by an experiment I made on an arch of
flame between the two poles of the great Voltaic apparatus of 2000 plates.
Platina melted with more facility in the arch at the positive than at the
negative extremity, and this arch was common air intensely ignited,
through which the electricity was discharged; and if any mechanical
current existed from the positive pole to the negative, the maximum of
heat must have been produced at the negative. When a wire of platina was
made positive, and brought in contact with charcoal rendered negative, it
became ignited much sooner, and fused into larger globules, than when made
negative, and brought in contact with the charcoal rendered positive; and
that the effect did not depend upon the greater heat of the charcoal,
appears from the circumstances, that similar phenomena occurred when the
experiment was made by contact with mercury. But when an imperfectly
conducting fluid, such as sulphuric acid, was used, the result was
reversed. The wire being negatively {130} electrified, and the acid
positively, the point in contact with the surface of the acid instantly
became white hot; in the opposite case a spark of blue light only was
produced.
The different appearance of the light on points positively and negatively
electrified, has been urged in favour of the idea of a fluid proceeding
from the positive to the negative surface. This phenomenon occurs as well
in the Voltaic, as in the common discharge: for when the arch of flame
passes between two points of charcoal, a vivid spot of white light is
always perceived on the negative point, and rays seem to diverge from the
positive point. The effect of the difference of the appearance of
differently electrified points, I find, does not depend upon the nature of
the elastic medium, for it takes place in hydrogen, carbonic acid, and
chlorine, though it is less distinct in the heavier gases, probably from
their being worse conductors; but the affections of light in passing from
different parts of the circuit, can with no more propriety be urged in
favour of a specific fluid, than the chemical changes produced by the
different poles.
When folds of paper are perforated by a discharge from an electrical jar,
there is a burr on both sides, which may be urged as an argument against
any fluid passing through; for it could penetrate in one direction only,
and the experiment is favourable to the idea that electricity is an
exhibition of attractive powers acting in peculiar combinations, for the
substance of the paper, which was negative, may be conceived violently
attracted to the positive surface, and the part which was positive, to the
negative, at the moment the discharge takes place.
It will be useless to pursue any further this recondite {131} part of the
subject; whatever view is taken, active powers must be supposed to be
bestowed upon species of matter, and the impulse must be ultimately
derived from the same source. In the universe nothing can be said to be
automatic, as nothing can be said to be without design. An imperfect
parallel may be found in human inventions; springs may move springs, and
wheels, indexes; but the motion and the regulation must be derived from
the artist; sounds may be produced by undulations in the air, undulations
of the air by vibrations of musical strings; but the impulse and the
melody must arise from the master.
Notes.
1. Plate II. fig. 8.
2. Plate II. fig. 9.
3. Plate II. fig. 10.
4. Plate II. fig. 11.
5. Plate III. fig. 12.
6. Plate III. fig.
13.
7. Plate III. fig. 14.
8. The conclusions are drawn from experiments made by the
electricity of the Voltaic apparatus.
9. See Plate III. fig. 14,
15.
10. Plate III. fig.
16.
11. [For an account of this battery and its effects, vide
Phil. Trans. for 1815.]
12. Plate III. fig.
17.
13. Plate IV. fig.
18.
14. Plate IV. fig.
19.




