 |
 |
 |
 |
 Chemistry's early history is nearly indistinguishable from alchemy, with which it shared both
presuppositions and methods. The first methodical treatise on
modern chemistry, in fact, by Andreas Libavius, bore the title
Alchemia (1597). But by the seventeenth century, chemists
proceeded largely empirically, systematically combining
compounds, studying the results of combustion and calcination,
and so forth. The invention of precise balances and scales in
the seventeenth and eighteenth centuries allowed for more
sophisticated research into the products of reactions.
Chemistry's early history is nearly indistinguishable from alchemy, with which it shared both
presuppositions and methods. The first methodical treatise on
modern chemistry, in fact, by Andreas Libavius, bore the title
Alchemia (1597). But by the seventeenth century, chemists
proceeded largely empirically, systematically combining
compounds, studying the results of combustion and calcination,
and so forth. The invention of precise balances and scales in
the seventeenth and eighteenth centuries allowed for more
sophisticated research into the products of reactions.In the seventeenth and eighteenth centuries, chemistry was concerned especially with questions of light, heat, and mechanics, topics largely picked up from Newton's Optics. Seventeenth- and eighteenth-century investigations into "airs" (such as Joseph Black's discovery of "fixed air," or carbon dioxide, in 1754) paved the way for phlogiston theory, the belief in a subtle substance that constituted the "fiery principle" in all combustible matter. Phlogiston theory slowly gave way over the course of the eighteenth century to the atomic theory that is accepted today.
One of the central figures in the rise of atomic theory was Joseph Priestley, who discovered oxygen and was able to demonstrate the absence of phlogiston in it.
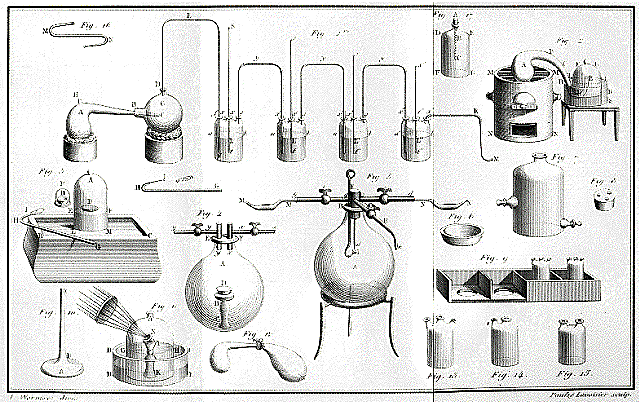
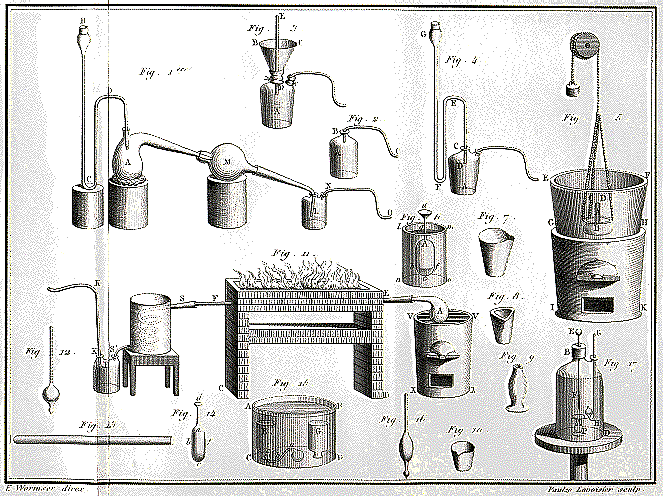 The work of Priestley, important as it was in its own right, is
especially valuable for paving the way for Antoine Lavoisier, the founder
of modern chemistry. It was up to Lavoisier to make sense of
Priestley's discovery of oxygen, which Priestley regarded as
"dephlogisticated air." Owing in part to his use of more precise
instruments than his predecessors, Lavoisier rejected phlogiston
theory in favor of an atomic theory of matter, and recognized
combustion not as the liberation of phlogiston but as the rapid
combination of the burning substance with the newly discovered
oxygen. Lavoisier's Traité élémentaire de
chimi (Elements of Chemistry, 1789) is often named as
the first modern chemical textbook. He identified twenty-three
elements (and was the first to define elements as substances
which could not be decomposed into simpler substances), giving
them their modern names, but he preserved the symbols of
traditional alchemy.
The work of Priestley, important as it was in its own right, is
especially valuable for paving the way for Antoine Lavoisier, the founder
of modern chemistry. It was up to Lavoisier to make sense of
Priestley's discovery of oxygen, which Priestley regarded as
"dephlogisticated air." Owing in part to his use of more precise
instruments than his predecessors, Lavoisier rejected phlogiston
theory in favor of an atomic theory of matter, and recognized
combustion not as the liberation of phlogiston but as the rapid
combination of the burning substance with the newly discovered
oxygen. Lavoisier's Traité élémentaire de
chimi (Elements of Chemistry, 1789) is often named as
the first modern chemical textbook. He identified twenty-three
elements (and was the first to define elements as substances
which could not be decomposed into simpler substances), giving
them their modern names, but he preserved the symbols of
traditional alchemy.
The first two decades of the nineteenth century in England constituted the most important era in the development of modern chemistry, with major implications for the science and technology of the industrial age that followed. The development of electrolysis (the decomposition of compounds into their elements with an electric current) opened the door to the discovery of new elements, and a number of chemists began to offer models of the nature of these elements. One of the leading investigators in this new field was Sir Humphry Davy, the most important chemist of his age, who describes his experiments with voltaic piles in his works. It was Davy who first argued that chemical bonds were essentially electrical in nature.
Another principal figure was John Dalton, who laid the foundation for modern chemical theory by resuscitating the classical atomic theory. Modern chemistry springs ultimately from a source in ancient Greece, when Democritus and Leucippus proposed an atomic theory of matter in the fifth century BCE, and in 1753, Henry Cavendish showed that water was the result of the union of hydrogen and oxygen. But atomic theory was given little scientific credence until Dalton's New System of Chemical Philosophy (1803), building on Lavoisier's findings, revived the theory and established modern quantitative chemistry. Dalton determined some atomic weights by assuming the simplest possible ratios of the numbers of atoms: having determined, for instance, that the ratio of the weights of hydrogen and oxygen, once they had been separated from water, was one to eight, he concluded that oxygen's atomic weight was eight. It was left for the French chemist Joseph-Louis Gay-Lussac to realize that each water molecule consisted of two hydrogen atoms to each oxygen atom, giving oxygen an atomic weight of sixteen.