 |
 |
 |
 |
In 1789, some time before he made the most important discovery, he was by accident led to the fact, of electricity having the property of exciting contractions in the muscles of animals. Stimulated by the then prevailing idea of electricity being a principal inherent in animals, which, acting upon the muscular susceptibility, was the immediate cause of muscular motion, he was induced to persevere in the inquiry, during the prosecution of which he brought to light other facts, which laid the foundation of this valuable scientific acquisition.
After having observed that common electricity, even that of lightning, produced vivid convulsions in the limbs of recently killed animals, he ascertained that metallic substances, by mere contact, under particular circumstances, excited similar commotions.
He found that it was essential, that the forces of metals employed should be of different kinds. He applied one piece of metal to the nerve of the part, and the other to the muscle, and afterwards connected the metals, either by bringing them together, or by connecting them by an arch of a metallic substance; every time this connection was formed, the convulsions took place. The diversity in the metals employed in these experiments appeared, in the very early stages of this inquiry, to be connected with their respective degrees of oxydability, the one being possessed of that property in a great degree, and the other little liable to the change. Hence zinc, and silver, or gold, was found to produce the greatest muscular contractions.
The experiments of Galvani were confirmed by many able philosophers, by whom they were repeated. Those who particularly distinguished themselves by their labours on this subject were, Valli, Volta, Drs. Monro and Fowler.
Galvani had theorised upon the phenomena, which he had observed to a considerable extent. He conceived, that the convulsions were produced by a disturbance of the electricity inherent in animals, which was identical with the nervous fluid, and that the metallic substances employed had not any other effect, than that of transmitting the electricity from the nerve to the muscles producing the contractions in question.
Simon Volta, with much labour and ingenuity, successfully opposed the hypothesis of Galvani. He had recourse to those valuable experiments made by Bennet, by which to explain the phenomena observed by Galvani. Bennet had some time before observed, when plates of different metals were brought in contact, that one of the metals transmitted a portion of its electricity to the other, each of which, when separated, being at the same time insulated, evinced signs of contrary states of electricity. When the plates, for instance, were one of copper and the other zinc, the former, while the two were in contact, gave a portion of its electricity to the latter. Hence, when they were separated, and thus presented to the electrometer, the copper exhibited signs of negative electricity, and zinc that of positive.
On this ground it was that Volta objected to the hypothesis of Galvani, and established the more plausible idea, that the electricity was furnished by the disturbance of that fluid, arising from the contact of the different metals, and that the convulsions were excited by the stimulating effect of that active agent. It was in the investigation of this experiment, that the truly ingenious philosopher was led to the discovery of the pile, which, from its inventor, has been called the Voltaic pile. This apparatus consisted, in combining the effects of a number of pairs of the different metals, and by that means constituting a battery in galvanism, similar in effect to the Leyden phial in common electricity.
As silver and zinc had been found in the minor experiments to produce the greatest effect, these metals were employed by Volta in the construction of his battery. The silver plates generally consisted of coins; and the zinc plates were of the same size, being frequently cast in moulds made with the silver. The same number of pieces of cloth, pasteboard, or leather, of the same size, and steeped in solution of common salt, were also provided. The above substances were formed into a pile, in the following order: zinc, silver, wet cloth; zinc, silver, wet cloth; and so on, in the same order, till the pile became sufficiently high. If it were to be elevated to any considerable height, it was usual to support it on the sides with three pillars of glass, or varnished wood.
The pile thus formed, was found to unite the effects of as many pairs of plates as might be employed. Previously to this, no other effect had been produced than what resulted from the energy of a single pair of plates. A pile of 50 pairs of plates, with as many corresponding pieces of wet cloth, was found to give a pretty smart shock, similar to an electric shock, every time that a communication was made between the top and bottom of the pile. It was found, however, that little or no shock was perceived, when the hands, or other parts applied, were not previously moistened. It was also observed that the effect was increased, when a larger surface was exposed to the action of the pile. If the communication were made by touching the pile with the tip of each finger merely, the effect was not perceived beyond the joint of the knuckle; but if a spoon, or other metallic substance, were grasped in moistened hands, the effect was felt up to the shoulder. If the communication be formed between any part of the face, particularly near the eyes, and another part of the body, a vivid flash of light is perceived before the eyes, corresponding with the shock. This phenomena may be more faintly observed, by placing a piece of silver, as a shilling, between the upper lip and the gum, and laying a piece of zinc at the same time upon the tongue: upon bringing the two metals in contact, a faint flash of light is perceived. It is singular, that this light is equally vivid in the dark with the strongest light, and whether the eyes be shut or open.
Another variety of galvanic battery was also contrived by Volta. The pairs of plates were soldered to each end of a bit of wire, which were afterwards bent into an arch, so that the plates became parallel to each other. A number of glass cups were also provided, and filled with a solution of culinary salt. The glasses being arranged side by side, the metallic arcs were so placed, that the silver plate was immersed into one glass, and the zinc in another; and also that a silver and zinc plate of different arcs should be placed in each glass. This arrangement was found to be similar to the pile, the water in the cups being substituted for the disks of cloth.
Soon after the discovery of the pile, in 1800, it was communicated by Volta himself to the Royal Society, London. The first experiments made in this country upon the Voltaic pile were made jointly by Messrs. Nicholson and Carlisle. After observing the phenomena already described by Volta, they observed an important fact, which had escaped the notion of that acute philosopher. When bringing the wire from the bottom of the pile, in contact with a drop of water at the top, they observed the disengagement of some gaseous substance, which had the smell of hydrogen. Supposing this effect to arise from the decomposition of the water, they caused the ends of two brass wires, coming from the two ends of the pile, to be immersed in water, so that a portion of the liquid might be exposed between the wires. A disengagement of gas immediately took place from one of the wires, while the other became as quickly tarnished and oxydated. The former appearance took place at the silver end of the pile, the latter at the zinc end. They ascertained, that the effect would not take place when the wires were placed far asunder, and that the effect diminished gradually with the distance. They observed also, that when the tincture of litmus was used, instead of water, the liquid in the vicinity of the oxydated wire, being that connected with the zinc end, became red. When they made use of wire of platina, instead of brass, they observed that the wire from the zinc end of the pile, which, when of brass, became oxydated, now gave out bubbles of gas, which they found to be oxygen. In short, they determined that the gases evolved were oxyden and hydrogen, and in proportions fit to constitute water. These discoveries established the chemical nature of the galvanic action in England; and they soon spread over all Europe.
The above experiments were repeated by Mr. Cruikshank, of Woolwich. He employed a glass tube, filled with water, having a cork at each end, through which wires of silver were passed, the points of which were separated from each other by a stratum of the liquid. Upon the wires being communicated with the two ends of the piles, the same appearances took place which were observed by Messrs. Nicholson and Carlisle: the silver wire, however, connected with the zinc end of the pile, became oxydated, the oxide forming a white cloud around the wire: he also, instead of water, introduced into the tube an infusion of Brazil wood. During the galvanic action, the colour in the vicinity of the wire of the zinc end became very pale, while that about the wire of the silver end of the pile appeared of a purple colour. When a metallic solution was placed in the tube, Mr. Cruickshank [sic] observed that, instead of hydrogen gas being evolved from the wire, which connected the silver end of the pile, as in the former experiments, the metal became revived.
He next caused the galvanic current to pass through solutions of the muriates of lime and soda. In these experiments, he found the oxygen evolved from the wire of the zinc end very deficient, and a smell of oxy-muriate produced. When gold wires were employed, the gold was dissolved by the oxy-muriatic acid., Aqua ammoniæ being operated upon in a similar way, both the water and the alkali underwent decomposition, producing the gases of hydrogen, nitrogen, and oxygen.
It is to the ingenious author of the above experiments, that we are indebted for the invention of the galvanic trough, a discovery which very soon superseded the use of the pile, as being more manageable, and attended with less trouble to the operator. It consist of a wooden box, or trough, the depth and breadth of which corresponds with the size of the plates. It is of such a length in general, as to contain fifty plates, allowing a space of about three-eights of an inch, between each pair of plates. The spaces between the plates are formed by grooves, which are to receive the plates. The plates are first soldered together in pairs, one of copper or silver, and one of zinc. The trough being lined with a cement, formed of bees'-wax and resin, the plates, which are previously warmed, are pressed into the grooves, in such order, that the zinc-side of each compound-plate may face one way, and the copper or silver the other.
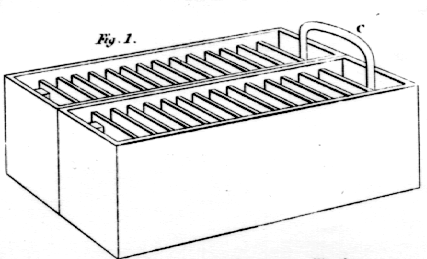
It will be easily perceived, that there is the strictest analogy between the trough and the pile, in point of arrangement. See fig. 1.
The pairs of plates of zinc and silver, which, in the pile, are simply laid upon each other, are, in the trough, soldered together, and cemented into the grooves; and the cavity or cells formed by the spaces between each pair of plates, in the trough, being filled with a solution of salt or other appropriate liquid, stands in the stead of the pieces of moistened cloth, between the plates of the pile.
Several powerful troughs were soon after constructed, the effects of which were strikingly evinced in producing other phenomenon, not as yet observed. Very small wires and foils of metal being exposed in their circuit, were deflagrated with great brillancy.
A number of galvanic experiments were made by Dr. Henry, of Manchester, in which he succeeded in decomposing the sulphuric and the nitric acids, and ammonia.
Mr. Davy, professor of chemistry at the Royal Institution, made a number of experiments, the most particular of which were those, in which he ascertained, that the dissimilarity of metals was not absolutely essential to the galvanic process. He succeeded first in exciting this energy by means of one metal, the two sides of which separated from each other. An oxydating liquid, such as an acid, was placed on one side of the plate, and a liquid having a contrary effect on the other. He afterwards produced an effect though more faintly, by treating plates of charcoal in a similar way. Hence it would appear, from these results, that the dissimilarity of the metals was only necessary to the furnishing two surfaces of different degrees of oxydability.
Hitherto it was not generally admitted, that the fluids of galvanism, and electricity, were identical. Dr. Wollaston made a number of experiments, which seem to have completely settled this point. He succeeded in decomposing water, by means of a current from the common electric machine. This effect, which had been performed with so much facility with the galvanic apparatus, was previously not know to be able to be produced by common electricity, and had hitherto appeared the most striking difference between the two principles.
This ingenious experimentalist, made a number of other experiments, tending to throw much light on the means of exciting and appreciating galvanic phenomena. He immersed each extremity of a piece of zinc and silver in dilute muriatic or sulphuric acid. The zinc, as would be expected, immediately caused the disengagement of hydrogen gas, while no appearance took place in the silver. As soon, however, as the two metals were made to touch each other at the opposite extremities, bubbles of hydrogen were copiously given out by the silver wire. Any other metal, capable of being acted upon by the acid, being substituted for the zinc, produced with the silver a similar effect. When gold was employed with silver, iron, or copper, in the dilute nitric acid, the same effect was produced; the gold being the same with the silver in the first experiment.
He made similar experiments, using metallic solutions instead of the dilute acid. Instead, however, of silver or gold giving out hydrogen gas, on the contact being made, the metal in solution became reduced. Thus, when iron and silver were placed in a solution of copper the iron immediately began to reduce the copper in solution, while the silver had not the slightest action. Upon bringing the two metals in contact, however, the silver became coated with copper. Dr. Wollaston attributes the curious phenomenon, above described, to a change of states in the electricity of the metals; and in order to confirm this idea, he attempted the same by means of common electricity, in which he succeeded to his utmost satisfaction. He supposes that the chemical affinitites are so altered by the the presence or absence of electricity, as to induce the anomalous appearances, which took place in the above experiments. The silver wire became coated with copper, and at the same time appeared to have the power of decomposing water.
The only mystery we observe in these experiments, is the liberation of the hydrogen in a situation where no oxygen is manifested either in the form of gas, or in any other state. Nor does the new doctrine, lately advanced by Mr. Davy, throw much light on the subject. The zinc in this experiment, is said to be positively electrified, and the copper of silver to be negative. That the zinc, on that account, attracts the oxygen of the water, and the silver the hydrogen. That the constituent parts of water are by the same law made to appear in situations where the decompostion did not take place, is very evident; hence it would appear that the hydrogen is carried by some means from the zinc to the silver. Or that the oxygen passes from the silver to the zinc, or according to Mr. Davy's hypothesis, the decomposition of the water takes places between the metals, the oxygen passing inevitably to the zinc, and the hydrogen in a similar way to the silver. To the latter there are several objections, which will appear from the following experiments.
Let a tube of three feet in length be filled with dilute muriatic acid, and corked at both ends, having a wire of zinc inserted in one end, and one of silver or platina in the other. The zinc will immediately begin to give out hydrogen, but no effect will be observed at the silver wire. Let a communication be established between the wires on the outside of the tube. The silver does not immediately give out bubbles, as was the case in the experiments of Dr. Wollaston, nor does that effect take place, till a few seconds after the contact of the metals. Can we for a moment suppose that the slight negative and positive electricity, produced by the contact of the two small wires, which would not affect the most delicate electrometer, can have the power, the one of attracting oxygen, and the other hydrogen, at the distance of eighteen inches, reckoning from the middle of the tube.
If the same tube be bent in the middle to an acute angle, like the letter V, according to Mr. Davy's hypothesis, the appearance of the hydrogen at the silver wire ought to take place as soon after the contact, as with the straight tube; but what is very singular, it will not take place at all. This experiment would seem to prove, that one of the constituents of water is carried through the whole length of the tube; and that by some law which differs from those of electricity, since the angle of the tube appeared to interrupt its passage. The interruption is still greater, even with a shorter tube, when the tube is bent in different places, forming a zig-zag.
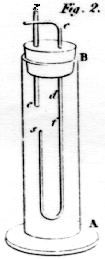 The idea that hydrogen
is carried from the zinc to the copper wire, is strongly favoured by
another experiment. Take the glass tube, AB, figure 2, filled with dilute
muriatic acid, having a cork at B, through which the wires, z and
c are passed, z being a wire of zinc, and c a wire of
platina, silver, or copper. So long as the wires remain unconnected at
z, the platina-wire appears unchanged; but, as soon as the contact
is formed, bubbles of hydrogen are first seen at d; they then very
slowly begin to appear in the lower parts of the wire; but what is
singular, the moment they begin to appear at f, they are also seen
at s, and some seconds are elapsed before any bubbles are seen at
g. If the hydrogen in the last experiment were attracted by the
negative state of the platina-wire, since the metal is the best conductor,
it would seem, that the point, s would be the last part to have
parted with its electricity; and, of course, the bubbles of hydrogen ought
to have appeared the last at that point, which is contrary to fact. It
therefore appears more likely that the hydrogen has been held in
combination by the electricity, the latter of which is taken by the
nearest metallic conductor in the circuit, leaving the hydrogen in its
gaseous form: the law, however, by which it moves along the liquid, does
not appear to agree with any known properties of electricity, since the
hydrogen is some seconds in reaching the point g.
The idea that hydrogen
is carried from the zinc to the copper wire, is strongly favoured by
another experiment. Take the glass tube, AB, figure 2, filled with dilute
muriatic acid, having a cork at B, through which the wires, z and
c are passed, z being a wire of zinc, and c a wire of
platina, silver, or copper. So long as the wires remain unconnected at
z, the platina-wire appears unchanged; but, as soon as the contact
is formed, bubbles of hydrogen are first seen at d; they then very
slowly begin to appear in the lower parts of the wire; but what is
singular, the moment they begin to appear at f, they are also seen
at s, and some seconds are elapsed before any bubbles are seen at
g. If the hydrogen in the last experiment were attracted by the
negative state of the platina-wire, since the metal is the best conductor,
it would seem, that the point, s would be the last part to have
parted with its electricity; and, of course, the bubbles of hydrogen ought
to have appeared the last at that point, which is contrary to fact. It
therefore appears more likely that the hydrogen has been held in
combination by the electricity, the latter of which is taken by the
nearest metallic conductor in the circuit, leaving the hydrogen in its
gaseous form: the law, however, by which it moves along the liquid, does
not appear to agree with any known properties of electricity, since the
hydrogen is some seconds in reaching the point g.
It will appear, from the above experiments, that the galvanic phenomena are essentially promoted, by having two metallic surfaces so situated, that one shall be oxydated, and that the other shall be situated as near it as possible, for the purpose of receiving its electricity. We have shewn, that the current is not only interrupted by distance, but that is essential the passage should be a direct line.
In Dr. Wollaston's experiments. when the wires were placed in a metallic solution, such as that of copper and silver, and the contact formed between the zinc and silver wires, no hydrogen was evolved by the latter, the contrary of which was the case with the dilute acid; but the metal in solution became reduced upon the silver.
There does not appear any thing mysterious in the reduction of the metal, since the hydrogen does not appear, bring employed in the deoxydation of the metal. A further proof that this is the case, is, that no other metals can be reduced in this way, but such as do not decompose water. This singular process enables us to account for several facts which have hitherto appeared anomalous. If a glass plate be smeared over with a solution of nitrate of silver, and a common pin be laid in the middle of the plate, beautiful ramifications of metallic silver will soon appear, as if vegetating from the pin. If the process be examined by a magnifying glass, the ramifications of silver may be fairly seen to grow from their ends. Though the more oxydable metal, the pin, may, in the first instance, have reduced a portion of silver, it does not account for the vegetative appearance which is afterwards observed. The pin cannot reduce the silver at so great a distance from itself, which is sometimes more than an inch. In order to prove, that the agency of the oxydable metal was not essential to the reduction of the metal, the writer of this article covered one half of the plate with liquid nitrate of silver, and the other half with dilute muriatic acid, suffering the liquids to touch each other; a wire of zinc was laid in the dilute acid, and one of platina in the nitrate of silver. As soon as the opposite ends of the wires were brought in contact, beautiful ramifications of silver soon began to appear from the platina wire, but no gas was observed.
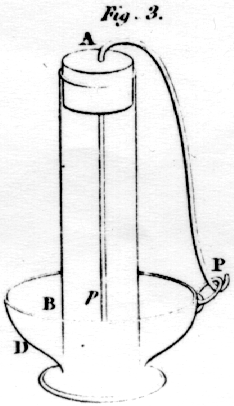 If a solution of
gold be used, instead of that of silver, the platina becomes speedily
gilt. The experiment producing what is called the lead-tree, cannot be
accounted for in any other way: it consists in filling a bottle with a
solution of acetate of lead, in the upper part of which is suspended a
piece of metallic zinc: in the course of a day or two metallic lead is
observed in shining filaments, suspended from the piece of zinc. The
same difficulty occurs in this, as in the last experiment: the filaments
of lead constantly grow from the ends at a distance of many inches from
the zinc. In order to prove that this experiment is similar to the
last, that is, that the lead is reduced by the hydrogen, take a tube,
AB, figure 3, at one end of which, tie a piece of bladder so tight that
the tube may hold water; let a cork be inserted at A, through which the
platina wire, Pp is passed; the tube being set up right in the
zinc cup, D, containing dilute muriatic acid, and a connection formed at
P, the platina soon becomes covered with brilliant crystals of metallic
lead: hence it would appear, that the platina had the power of reducing
the lead into its metallic state, or that some substance had been
transmitted through the bladder adequate to that effect. If, instead of
the acetate of lead, the tube be filled with dilute acid, upon the
connection being formed at P, the platina becomes covered with bubbles
of hydrogen: need we, therefore, hesitate in concluding, that the lead
owes its reduction to the hydrogen.
If a solution of
gold be used, instead of that of silver, the platina becomes speedily
gilt. The experiment producing what is called the lead-tree, cannot be
accounted for in any other way: it consists in filling a bottle with a
solution of acetate of lead, in the upper part of which is suspended a
piece of metallic zinc: in the course of a day or two metallic lead is
observed in shining filaments, suspended from the piece of zinc. The
same difficulty occurs in this, as in the last experiment: the filaments
of lead constantly grow from the ends at a distance of many inches from
the zinc. In order to prove that this experiment is similar to the
last, that is, that the lead is reduced by the hydrogen, take a tube,
AB, figure 3, at one end of which, tie a piece of bladder so tight that
the tube may hold water; let a cork be inserted at A, through which the
platina wire, Pp is passed; the tube being set up right in the
zinc cup, D, containing dilute muriatic acid, and a connection formed at
P, the platina soon becomes covered with brilliant crystals of metallic
lead: hence it would appear, that the platina had the power of reducing
the lead into its metallic state, or that some substance had been
transmitted through the bladder adequate to that effect. If, instead of
the acetate of lead, the tube be filled with dilute acid, upon the
connection being formed at P, the platina becomes covered with bubbles
of hydrogen: need we, therefore, hesitate in concluding, that the lead
owes its reduction to the hydrogen.
The method of whitening brass and copper, by boiling them with cream of tartar and tin, is a process of this kind; the cream of tartar, and the metallic tin, answering the purpose of the zinc and acetate of lead, in the last experiment: a portion of the tin in solution is reduced upon the copper or brass, rendering it white, by the hydrogen which is produced during the galvanic contact of the copper or brass, with the tin.
In all the experiments, the zinc wire is, during its contact with that of the platina, silver, &c. undergoing an increased oxydation, which is proportionate to the quantity of hydrogen evolved at the platina wire; since the oxygen of that, and hydrogen, both of which are derived from the water, are disposed of in the oxydation of the zinc. The hydrogen passes from the zinc to the opposite wire, with the greatest facility, through a direct liquid communication, the shorter the better. It becomes much interrupted by having to turn sharp angles, or in passing through small apertures. It passes with more or less freedom through solid bodies, when moistened with water, but does not pass at all, except when moisture is present.
Having given an account of the effects resulting from a single galvanic combination, we will next give some account of the constructions of that compound apparatus, termed Galvanic, or more properly, the Voltaic battery.
The pile of Volta, of which we have already given a slight description, is at present so little used, that we shall direct our attention more particularly to the trough, as being more convenient for experiments than the pile, and at the same time less liable to be out of order.
The wood of which the trough is formed, should be the oldest and hardest mahogany, being less liable to warp than other kinds of wood. The sides of the trough must be dove-tailed together, and the bottom ought to be grooved into the sides, and fitted-in with turpentine; perpendicular grooves must be made in the sides of the trough, for the reception of the plates, correspondent to which there must be grooves in the bottom. When the length of a trough is more than two feet, it becomes unwieldy; it should not even be that length, when the size of the plates would render it too heavy to be handed about. The distance between the plates should be about three-eights of an inch; if they are nearer together, the acid employed is too soon exhausted, and, consequently, the power of the battery less lasting.
The plates should be of copper and zinc. Though silver is stronger than copper, it is not so in proportion to the price.
The zinc plates are best cut out of sheets of malleable zinc, as being cheaper, less liable to break, and may be used much thinner.
The copper may be employed so thin as six ounces to the square foot.
The plates of copper being made a little larger than the zinc, may be lapped over the edges of the latter, by which means they fit much closer to the zinc plate, without the labour of hammering the copper plates previously flat. The copper plates only require to be soldered to the upper edge of the zinc plate, since the other three edges are so secured with cement in the grooves as to preclude the necessity of soldering. The lapping over of the copper is sufficient to keep it close to the zinc plate till the plate is fastened in the trough. Previously to inserting the plates in the trough, the inside must be lined with a cement, formed of resin and bees-wax, or what is cheaper, of six parts of resin and one of lime and oil. The plates, being previously warmed, are to be pressed down into the grooves before the cement becomes quite cold. After the plates have been inserted, in such order that all the zinc surfaces shall face one way and the copper the other, the cement must be more evenly adjusted with a hot iron which will reach to the bottom of the cells; the trough being laid first on one side and then on the other for that purpose.
When the cementing process is finished, and the whole sufficiently cold, the trough must be dressed off and varnished with copal varnish where it can be had; but in lieu of that with common spirit varnish. When the varnish is dry it must be polished with rotten-stone and water.
In the above construction it is manifest that two of the surfaces are lost by being laid and soldered together. About two years ago the writer of this article had conceived the possibility of making use of both the surfaces of the copper and zinc plates at the same time. Accordingly he cemented into a trough, in the groove made for the plates of metal, plates of glass. The metal plates were formed by soldering together a plate of each, of copper and zinc, and then bending them till the plates became parallel to each other, leaving a space between the two surfaces a little wider than the thickness of the glass plates.
The cells between the glass plates being filled with the proper liquid, each of the above compound plates were made to bestride one of the glass plates, in such order than a zinc and copper plate of two different compound plates, in succession to each other, may occupy each of the cells. All the surfaces are by this contrivance exposed to the action of the liquid, and might be considered double the power of a common trough, having the same number of plates.
Little or no advantage was gained by this method. Though there are two surfaces of each metal in each of the cells, it will be evident, from several minor experiments already given, that two of the surfaces are so completely disconnected as to produce little or no effect. One of the zinc surfaces in this trough is facing the glass on one side the cell, and one of the copper surfaces is similarly situated on the other side.
The trough, therefore, which is represented in figure 1, and which has been particularly described, is, for general use, the most convenient, and in other respects, the best battery yet produced.The next thing to be considered, is the management of the galvanic battery. First, all of the cells of the trough must be filled, within about half an inch of the top, with a liquid, composed of water, with about one twenty-fifth part of the muriatic or the nitric acid. The plates of the trough are shorter than the depth of the trough, by about three-quarters of an inch; so that the trough may be leaned on one side in the filling, for the purpose of letting the liquid run equally into all the cells.
If a number of troughs are to be connected together, the communication must be made by arcs of metal, which are inserted into the liquid of one cell of each trough as represented in fig. 1, at C. In making the connection, it is to be observed, that the zinc surface of one trough must correspond with the copper one of another, and the zinc of the latter with copper of a third, and so on. This arrangement may be better conceived by placing them in the same order, and to end in such a way, that all the zinc surfaces may face one way, and the copper ones the other. After all the troughs are connected together, let the two unconnected ends, at which the experiments are to be made, be as near as possible.
A connection being now formed between the two ends, one of which we shall term the zinc end, and the other the copper end, the united energy of the whole will be transmitted through the connecting medium.
EXPERIMENTS.
The most striking and the most common experiments are those which consist in the galvanic energy upon the organs of animals. If two metallic rods, or, what is equally convenient, two silver spoons, be grasped, one in each hand, the skin of the part being previously moistened with a solution of salt, and one of the spoons be brought in contact with one end of the battery, the moment the other comes in contact with the other end of the battery, the shock is perceived. Fifty compound plates will give a shock which will be felt in the elbows. One of a hundred will be felt in the shoulders. A greater number of plates give so forcible a shock to the muscles, as to be dreaded a second time. The shock appears to depend upon the number of plates. The stun, or first impression, is much the same, whatever may be the size of the plates; at least, from the size of two inches square to that of ten; the surfaces being at four to one hundred. The effect upon the muscles, as well as upon the cuticle itself, is very different from large plates, when the series is the same. It appears, that the shock, or first impression, is as the series, which is also as the intensity of the electricity. If the shock be received from the same number of large plates, the same species of commotion is produced in the first instance, as with the small plates; but if the contact be still kept up, a continuation of the effect is perceived, which is felt through the whole arms, producing a vast tremor, attended with a sensation of warmth. If the plates be from eight to twelve inches square, this effect may be perpetually kept, while the acid in the cells is expended.
Though small plates have been recommended for medical purposes, we think large ones will be found more likely to have a good effect. If the medical advantage is to be derived from the stimulus of galvanism, the effect of a perpetual and regular current of the stimulus must certainly be preferable to the rapid transmission of a small quantity.
The galvanic shock may also be conveniently given, by immersing the hands or the feet into vessels contained a solution of salt, and bringing wires from each end of the battery into the liquid. If any other part of the body is intended to be operated upon, a sponge, moistened with salt water, fastened to a metal plate connected with one end of the battery, may be applied to the part, and the hand or foot put into a vessel of the same liquid, connected by a wire with the other end of the battery. Small bits of sponge or bits of leather may be fastened to the end of the connecting wires. and made more or less moist as the delicacy of the part may require. This contrivance is very useful in operating upon the eyes or ears.
When galvanism is used medically, it should first be applied very feebly, and the effect gradually increased, as the susceptibility of the part will admit. If the part has, from disease, become so languid and insusceptible, as not to be sensible of the effect, it should be scarified, or by other means have the cuticle removed. This is sometimes the case with languid tumors, and some cases of paralysis. Though we had no great opinion of the medical agency of galvanism, we have lately heard of several very successful cases, one of which in particular was the cure of perfect loss of speech. If the naked metal of the wire, from a powerful battery, be applied to the skin, it becomes cauterized and black.
If the plate, covered with a moistened sponge, connected with one end of the battery, be applied to the back of the head, at the same time that the moistened fingers of one hand are slightly applied to the other end, a smarting sensation will be felt in the part, and a taste at the same time will be felt in the mouth, similar, but in a greater degree, to that occasioned by the piece of zinc, and the shilling when laid upon the tongue. This experiment succeeds the best with a small number of large plates, as much as ten inches square.
Decomposition of Water and other Bodies.
The most simple way of performing this experiment is to bring the wires coming from each end of the battery into a vessel of water. A profusion of bubbles of gas will appear to be given out from each wire, as far as they are immersed in the liquid. The nearer the wires are brought together, so as not to touch, the more rapidly the decomposition goes on. The gas produced from the wire coming from the zinc end of the battery, if the wire be gold or platina, is found to be oxygen gas; but if the wire be of any more oxydable metal, no gas will appear, but the wire becomes oxydated. The gas furnished by the wire from the copper end of the battery, of whatever kind of metal the wire may be, is pure hydrogen. If the immersed part of this, however, be previously oxydated, no gas will be observed for some time, the hydrogen being employed in reducing the oxide upon the surface.
Both the gases are furnished from the decomposition of the water.
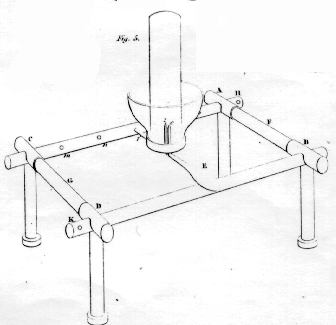
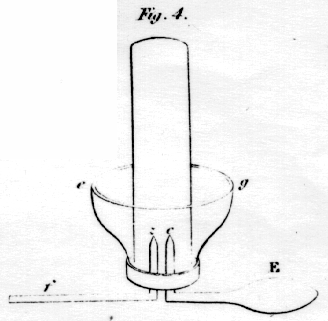 An apparatus more
convenient for this experiment, and at the same time fitted for
collecting gases is shewn in fig 4; cg, is a cup of glass capable
of receiving the glass tube, h; Ec, and fz, are two
wires of platina, fitted into two holes perforated in the bottom of the
glass cup; the tube, h, which is close at the top, is first
filled with the water or other liquid, and the cup inverted upon it; the
whole are then suddenly returned into their erect position: this
apparatus is then placed in the frame, fig 5; ABCD are four pieces of
brass, united together by the pieces of glass, F and G, and supported by
four legs, through which also the brass rods, H and K, are passed. It is
plain, the two sides of this frame are insulated with respect to each
other, at least as much as is necessary for any galvanic experiment.
The part f, in fig. 4, being introduced into any of the holes,
such as nm, the opposite end, F, is made to rest on the opposite
brass rod, K. If the wires from the battery be now conected with the
frame at H and K, the gas will instantly begin to rise from the wires,
c and z, up into the tube, while the liquid descends and
occupies the cup.
An apparatus more
convenient for this experiment, and at the same time fitted for
collecting gases is shewn in fig 4; cg, is a cup of glass capable
of receiving the glass tube, h; Ec, and fz, are two
wires of platina, fitted into two holes perforated in the bottom of the
glass cup; the tube, h, which is close at the top, is first
filled with the water or other liquid, and the cup inverted upon it; the
whole are then suddenly returned into their erect position: this
apparatus is then placed in the frame, fig 5; ABCD are four pieces of
brass, united together by the pieces of glass, F and G, and supported by
four legs, through which also the brass rods, H and K, are passed. It is
plain, the two sides of this frame are insulated with respect to each
other, at least as much as is necessary for any galvanic experiment.
The part f, in fig. 4, being introduced into any of the holes,
such as nm, the opposite end, F, is made to rest on the opposite
brass rod, K. If the wires from the battery be now conected with the
frame at H and K, the gas will instantly begin to rise from the wires,
c and z, up into the tube, while the liquid descends and
occupies the cup.
A number of the apparatus, such as fig. 4, may be employed at the same time; and if the different tubes are filled with different liquids, such as the various solutions of salts, and the communication of each occasionally cut off, by placing some non-conductor at E, their relative conducting powers may be ascertained.
If two tubes of smaller size be placed, one over the wire, z, and the other over that of c, the gases may be collected separately.
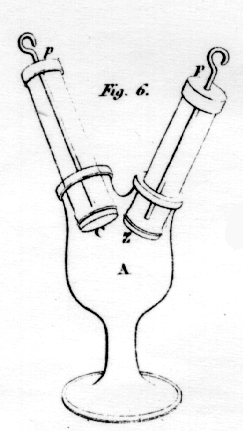 If the tube contains
a metallic solution, such as silver, lead, or copper, the wire from the
copper end of the battery will afford no gas; but the metal of the
solution will be reduced. Let the glass vessel A, fig. 6, have the two
tubes, z and c, ground into its two necks. At the ends,
z and c of the tubes, are tied bits of bladder, so that
any liquid in the tubes may have no tendency to enter the vessel A. The
vessel being previously filled with some liquid, the tubes are inserted
that no air may exist between the ends of the tubes; the tubes are also
provided with two small caps of ivory or wood, through which the platina
wires, pp, are passed, reaching the bottom so near as not to
pierce the bladders. The tubes being filled with water, and the wire
from the zinc-end of the battery connected with the wire of tube
z, while that of the copper is attached to that of tube c,
the decomposition of water will speedily commence, the wire in z
affording oxygen gas, while that of c affords hydrogen gas. In a
very short time, the liquid of the tube, z, will be found to
contain muriatic acid; or rather, the oxy-muriatic; and the tube,
c, will at the same time be found to contain a fixed alkali. if
the tubes be filled with infusion of cabbage, the signs of alkali and
acid are very soon observed, from the liquid of z becoming red,
and that of c green. If the connection be reversed, the liquids
repass to the blue colour, and if the process be continued, that of
z becomes green, and c red.
If the tube contains
a metallic solution, such as silver, lead, or copper, the wire from the
copper end of the battery will afford no gas; but the metal of the
solution will be reduced. Let the glass vessel A, fig. 6, have the two
tubes, z and c, ground into its two necks. At the ends,
z and c of the tubes, are tied bits of bladder, so that
any liquid in the tubes may have no tendency to enter the vessel A. The
vessel being previously filled with some liquid, the tubes are inserted
that no air may exist between the ends of the tubes; the tubes are also
provided with two small caps of ivory or wood, through which the platina
wires, pp, are passed, reaching the bottom so near as not to
pierce the bladders. The tubes being filled with water, and the wire
from the zinc-end of the battery connected with the wire of tube
z, while that of the copper is attached to that of tube c,
the decomposition of water will speedily commence, the wire in z
affording oxygen gas, while that of c affords hydrogen gas. In a
very short time, the liquid of the tube, z, will be found to
contain muriatic acid; or rather, the oxy-muriatic; and the tube,
c, will at the same time be found to contain a fixed alkali. if
the tubes be filled with infusion of cabbage, the signs of alkali and
acid are very soon observed, from the liquid of z becoming red,
and that of c green. If the connection be reversed, the liquids
repass to the blue colour, and if the process be continued, that of
z becomes green, and c red.
Galvanism, as a source of light and heat.
Batteries of great dimensions, such as contain from 5,000 to 10,000 square inches each, of zinc and copper surface are capable of furnishing abundance of sensible heat and much light. If the connection between the two ends of the battery be made by a very small wire, such as the fine watch-spring wire, the wire becomes red-hot for a considerable length, and if the power of the battery be great, it becomes white-hot and ultimately fused.
Let the end of the wires of the battery be each provided with a pair of tweezers, one pair of which being insulated from the hand by covering the surface with dry cloth; place between each pair of tweezers a small bit of charcoal, made in a close vessel, from boxwood, or lignum vitæ. The moment the contact is formed between the bits of charcoal, a vivid light is produced, much more brilliant than that occasioned by burning in oxygen. If the contact be frequently severed by a sort of tremulous motion, the light may be kept up for some time.
The foils and small wires of metals are deflagrated by placing them in the current. Let one of the conducting wires be brought in contact with an iron dish, filled with mercury. Let the foil or small wires be attached to the other conducting wire, and be brought in contact with the surface of the mercury, which constantly presenting a clear surface, is very convenient in these experiments. A very brilliant effect may also be produced, by presenting the foils to the surface of a sheet of tinsel.
In inflaming oils, alcohol, &c. by galvanism, some thin metallic substance, or a small piece of charcoal, should be covered with the substance to be inflamed. The moment the contact is made, as in deflagrating the metal, the oil takes fire.
The galvanic spark, with great facility, fires a mixture of oxygen and hydrogen gases.
A very brilliant discovery has lately been made by Mr. Davy, Professor at the Royal Institution, and confirmed by others, which consists in the decomposition of the two fixed alkalies. It is performed by placing a bit of alkali in the solid state, and a little moistened, upon a plate of platina, connected with one end of the battery, and bringing into contact with it another piece of platina, from the other end of the battery. A portion of black matter is soon formed, in which is found imbedded small metallic globules; which substance is found to be the base of the alkali, and has been deprived of its oxygen by the galvanic agency. These globules are so imflammable as to decompose water, with a brilliant flash and slight explosion.
This discovery will be of great importance to chemistry, and will probably soon make a serious change in its arrangement and nomenclature.